“Using patient specific imaging data, we calibrated our biology-based, mathematical model to make predictions of how tumors grow in space and time,” Yankeelov said. “These predictions have shown to be highly accurate when predicting the response of triple negative breast cancer patients to standard neoadjuvant chemotherapy.” This type of chemotherapy is widely accepted as the standard-of-care for early TNBC, but it comes with concerns of clinical benefits versus harm from the treatment.
Improved predictions provide physicians with guidance on whether a particular treatment is likely to work. “If our model predicts that the treatment is going to be beneficial, then they have more confidence staying the course with chemotherapy. Conversely, if our model predicts that the treatment is not going to be beneficial, then they have more confidence finding an alternative intervention,” Yankeelov said.
Yankeelov’s mathematical models describe how tumor cells change in space and time due to factors such as how the cells migrate, how they proliferate, and how they respond to therapy.
“What we do is make MRI measurements that let us calibrate those model parameters based on an individual patient's MRI measurements," Yankeelov said. "Once the model is calibrated, we run it forward to predict how that patient's tumor will grow in space and time — this prediction can then be compared to actual measurements in the patient at a future time. It is these predictions that we are getting surprisingly good at.”
Going forward, his lab’s goal is to go beyond making a prediction of whether a patient will respond to therapies or not. Instead, it is about using mathematical modeling to identify an optimal intervention strategy.
“If you have a model that can accurately predict the spatial and temporal development of a tumor, then we use a supercomputer to try an array of treatment schedules to identify the one that works best. That is, we use the mathematical model to build a 'digital twin' to try a myriad of treatment schedules to identify the one with the highest probability of success. That is where the research and field is going,” Yankeelov added.
Yankeelov’s lab used TACC’s Stampede2 supercomputer and Corral high performance storage in developing digital twins. It's a fast turnaround — the goal is to get the digital twins to work within 24 hours of getting a patient's data to help a physician with treatment decisions within 24 hours, according to Yankeelov. To reach that goal requires access to a supercomputer.
"Over the last eight years, TACC has provided extensive computational support for our research efforts via Lonestar5, Lonestar6, and Frontera," Yankeelov said. "Indeed, it started within the first weeks of our arrival in Austin where TACC staff visited our lab to provide a rapid tutorial on how to start using the systems. TACC has been there every step of the way as we develop methods for improving the treatment of — and outcomes for — patients battling cancer."
HER2+ and Combined Therapies
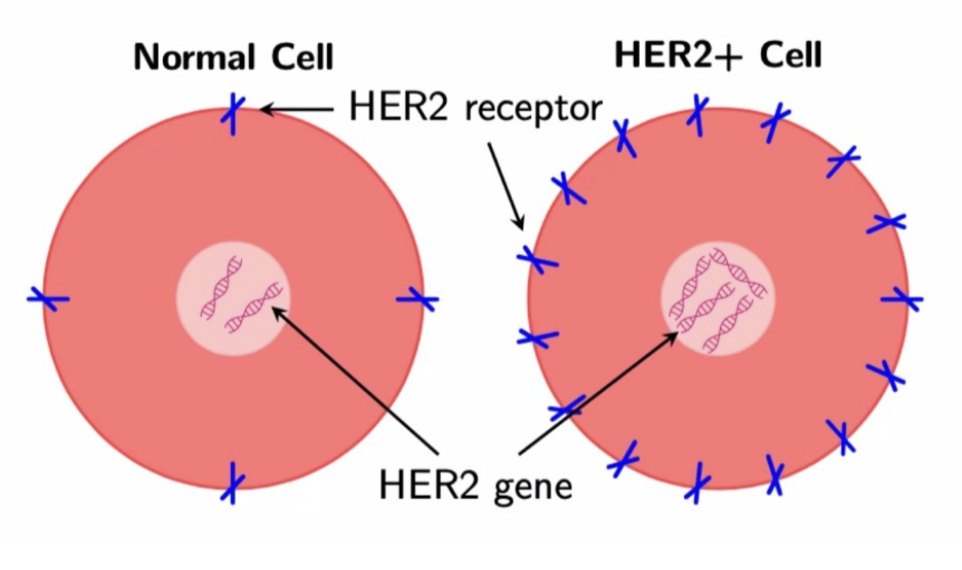
HER2+ breast cancer overexpresses the gene that makes the HER2 protein — it is characterized as an aggressive breast cancer that can respond well to treatments such as Trastuzumab (a monoclonal antibody), which typically is administered in combination with Doxorubicin (a chemotherapy drug). The challenge for researchers and physicians lies in optimizing the combination of these two drugs to maximize treatment efficacy.
“I developed several mathematical models to assess their ability to replicate experimental data with mice receiving various drug combinations obtained by our collaborator Anna Sorace,” said Ernesto Lima who is a Research Associate at the Oden Institute’s Center for Computational Oncology.
Lima co-authored along with Yankeelov a 2022 study published in Computational Methods in Applied Mechanics and Engineering. It developed a family of models to capture the effects of combination Trastuzumab and Doxorubicin on tumor growth to optimize the outcome of the combination therapy while minimizing the dosage and thereby the toxic side-effects necessary to achieve tumor control.
“We created 10 models and calibrated them using the experimental data," Lima said. "Calibration involves adjusting parameters, such as the proliferation rate, which dictates how fast the tumor volume increases over time to align the model's output with the experimental data."