“Being awarded this grant is significant encouragement for not only our proposed research but also the vision of the Oncological Data and Computational Science collaboration between MD Anderson, TACC, and the Oden Institute,” said Hormuth.
“As an engineer who is striving to improve cancer care, these types of collaborations are central to understanding the unmet clinical needs, barriers to implementation, and complexities of the disease itself.”
With the mathematical strategies of computational oncology, the balance between efficacy and toxicity during cancer treatment can be predicted and controlled - delivering personalized medicine to each and every patient.
This project is focused principally on radiotherapeutics as a cancer treatment. However, efficacy and toxicity are two fundamental parameters that determine the fate of any therapeutic. Efficacy describes the best possible response that treatment can elicit. Higher treatment dose packs more of a punch, increasing the efficacy of cancer treatments such as chemotherapy and radiotherapy. However, excessive radiation or drug concentration spikes unacceptable levels of toxicity, poisoning the patients it was designed to help. To complicate matters further, drug concentrations in cancer treatment are typically predetermined by the result of widespread clinical trials which often underrepresent racial and ethnic minorities, and older, rural, and lower-income Americans.
With the help of the CPRIT grant, Chung and Hormuth can further develop their mechanistic models of tumor growth, incorporating patient-specific data to predict response prior to the completion of treatment to enable pre-emptive personalized and adaptive targeting of radiotherapy. This could allow oncologists to provide patients with treatments reflecting the ideal efficacy-to-toxicity ratio for each, without putting them through a draining trial run of experimentation and adjustment.
“This project couldn’t be attempted without close collaboration between engineers and clinicians,” explained Hormuth.
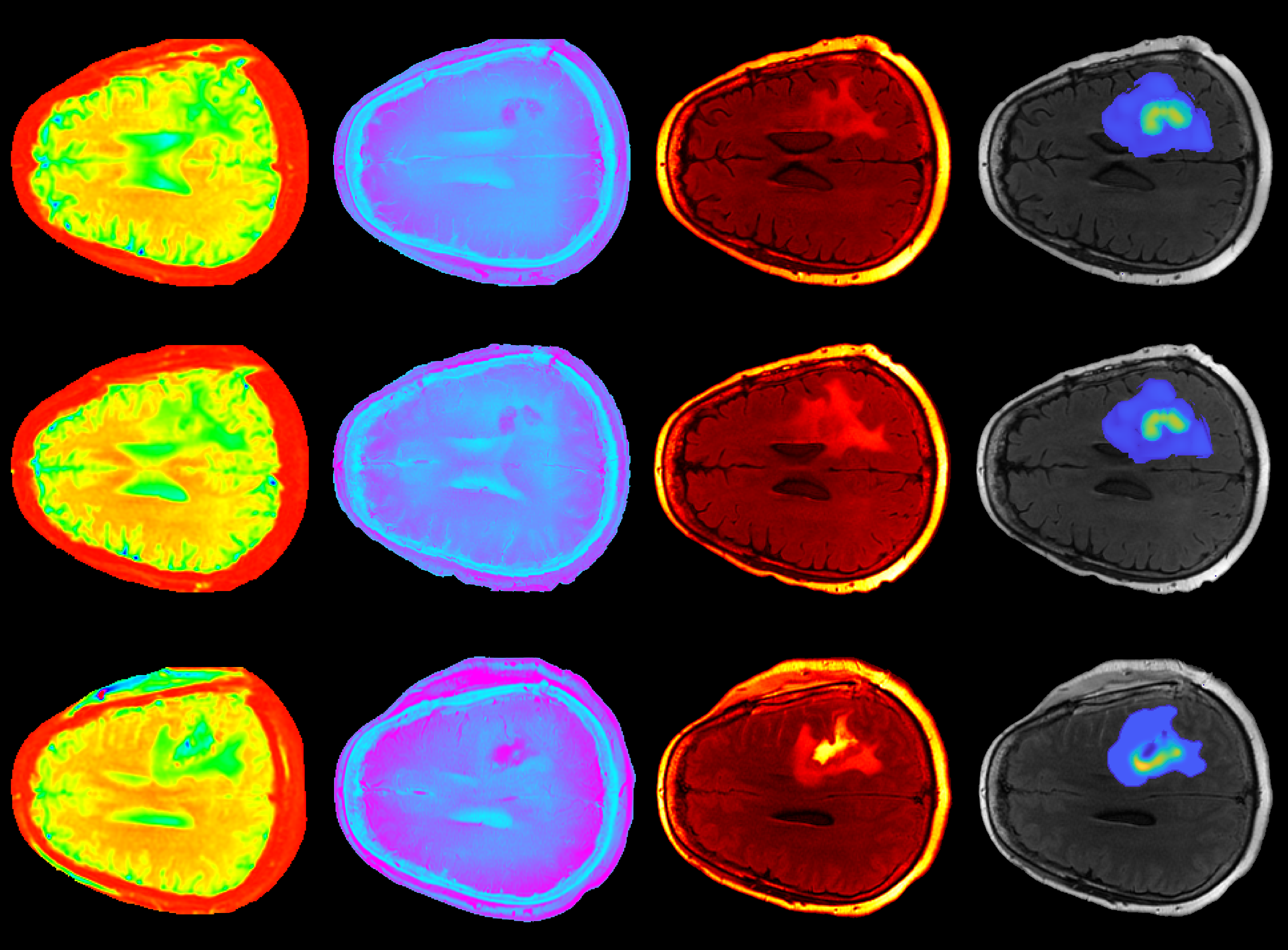
Together, Hormuth and Chung have merged various quantitative imaging measurements with computational simulations to create an accurate model for calculating the progression of high-grade glioma, a type of tumor that occurs in the brain and spinal cord.
Central to their approach is the use of imaging data sensitive to underlying structure and biology of the tumor. One approach, diffusion tensor imaging, measures the diffusion of water molecules in the brain to provide detailed information about the microstructure of neural tissues. This information ranges from fiber orientation (a type of white matter tract that connects the cortex with other areas, such as the brainstem and spine) to the degree of myelination (the process by which the brain produces layers of sheaths that wrap around nerves and act as layers of insulation). The careful observation of such variables can reveal the existence and effect of a high-grade glioma on the brain.
“With this CPRIT support, our research will provide tangible steps toward utilizing the rich imaging data in combination with mathematical modeling to deliver personalized, anticipatory treatment based on predictions at an individual patient level,” said Chung. “As a practicing radiation oncologist, I believe this is a transformative step toward how we target tumors.
“The research funded here reflects the innovative and collaborative vision shared by the dedicated researchers at MD Anderson, UT Austin’s Oden Institute and the Texas Advanced Computing Center to develop new approaches to end cancer,” said Dr. David Jaffray, Chief Technology and Digital Officer at MD Anderson.
“Together, we are working at the transformative intersection of computing and data science to push us further into the future of cancer care and discovery.”